Casual Tips About How To Find Out Number Of Electrons

This is why we have the 2 n 2 rule.
How to find out number of electrons. Atoms have 1 or 2 electron seashells, and the number of electron seashells is determined by the group of the atom. You can use these numbers to calculate the number of. Find how many valence electrons the.
How to calculate the number of electrons? So from figure 3, the number of electrons for chloride ion is 17 + 1 = 18 electrons. The outermost shell of nitrogen has (2s 2 2p 3 ), therefore, the total.
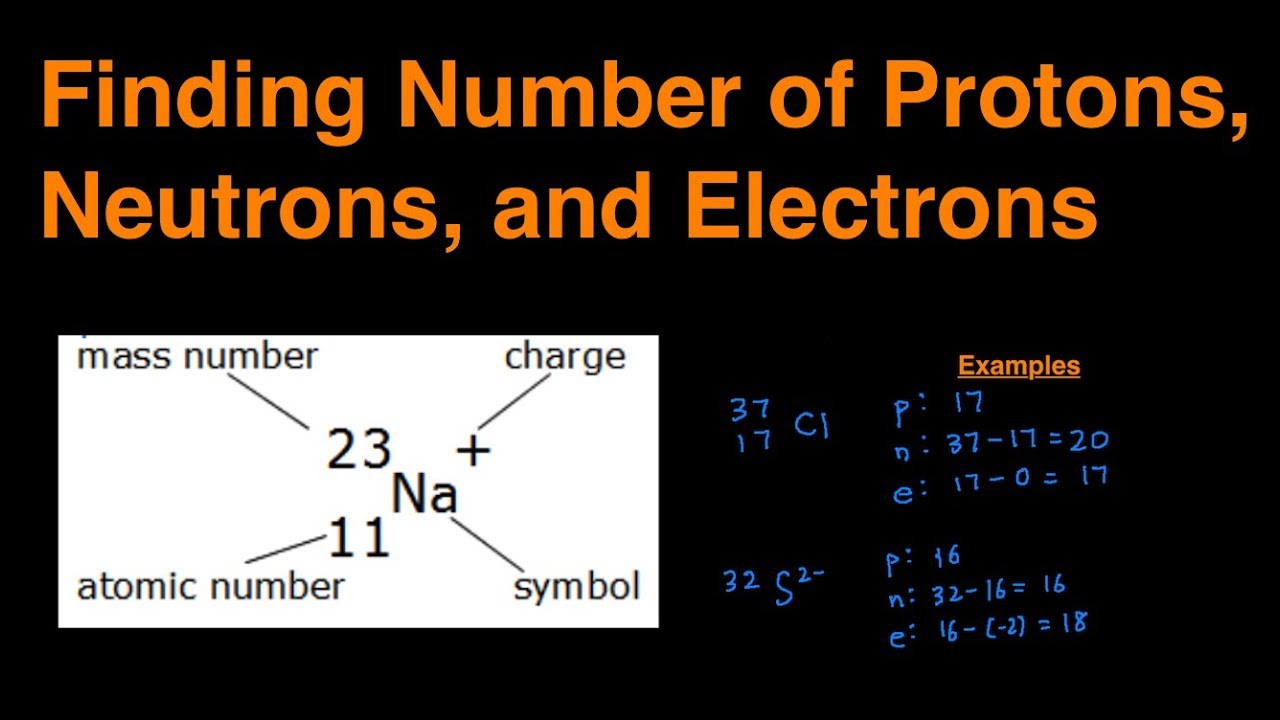
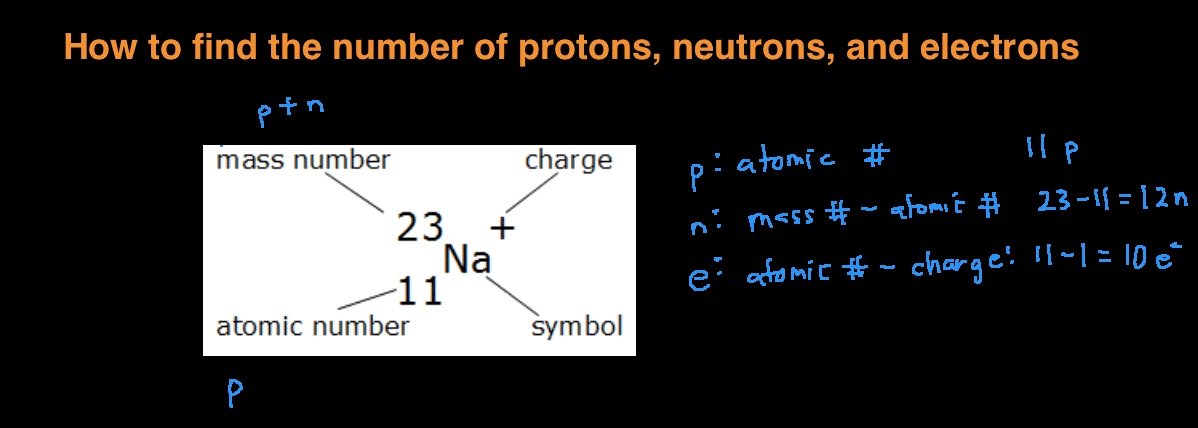
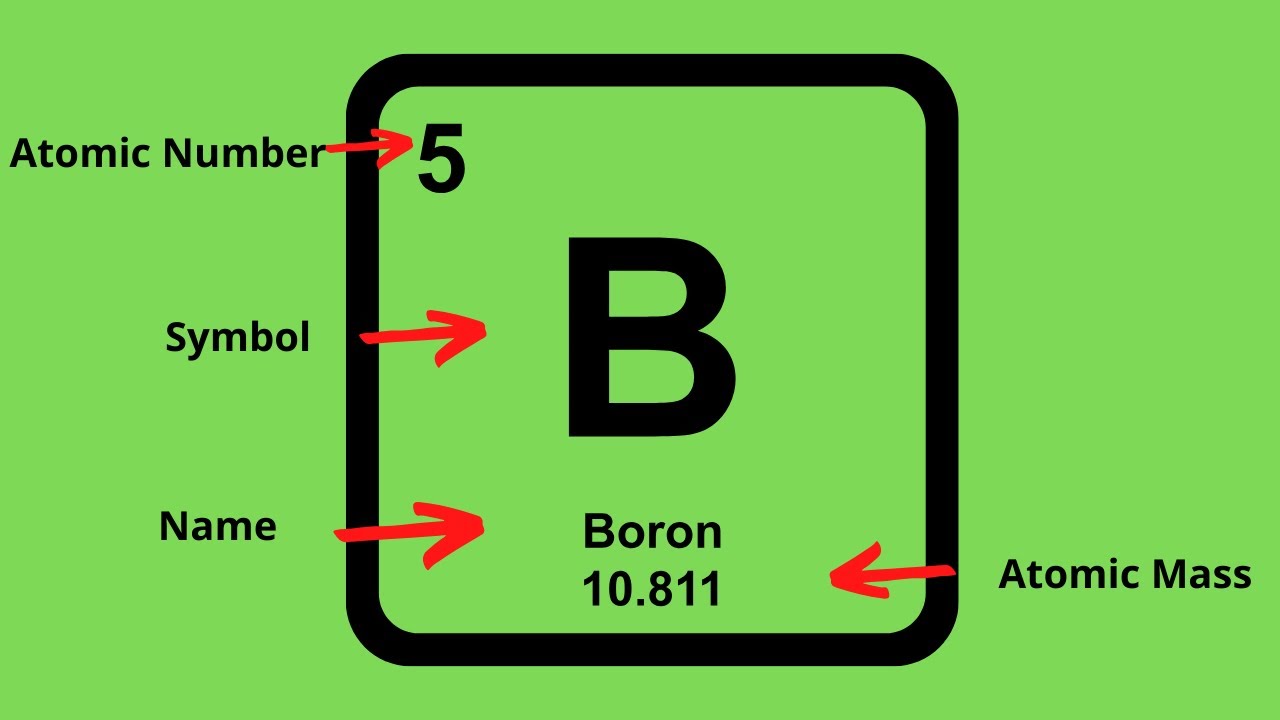
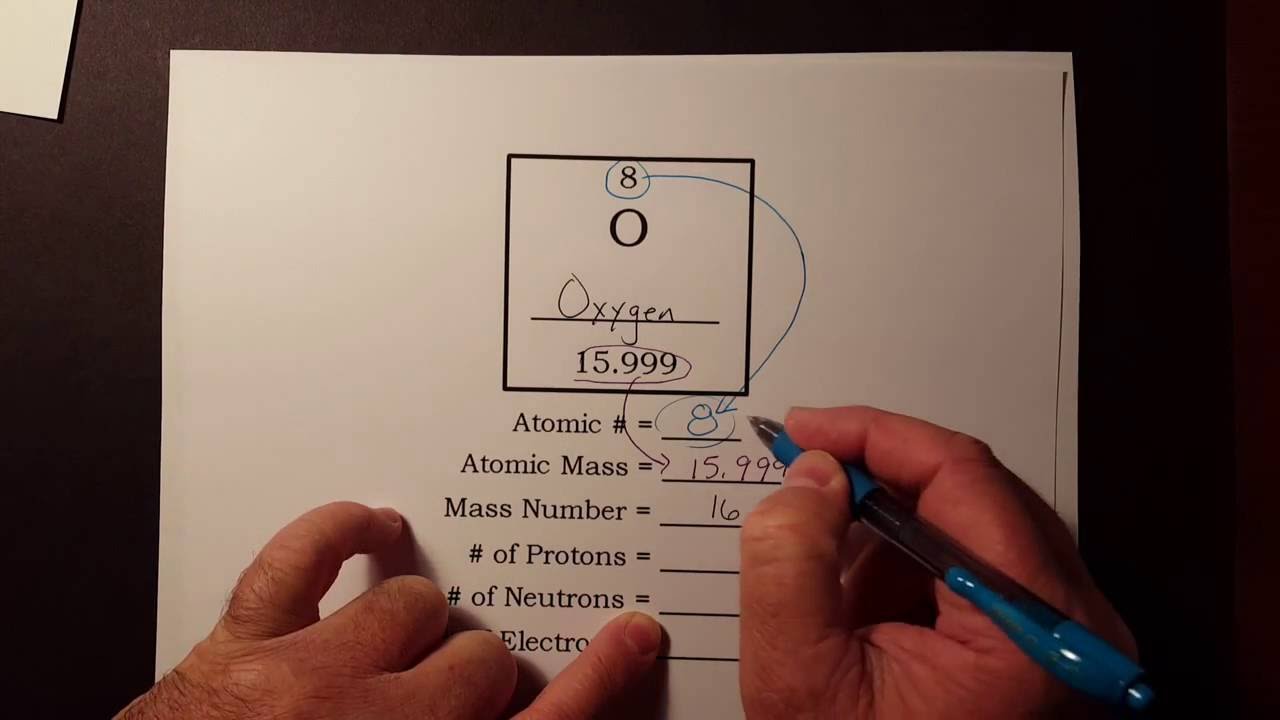
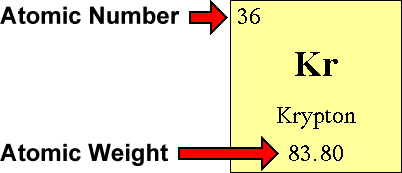
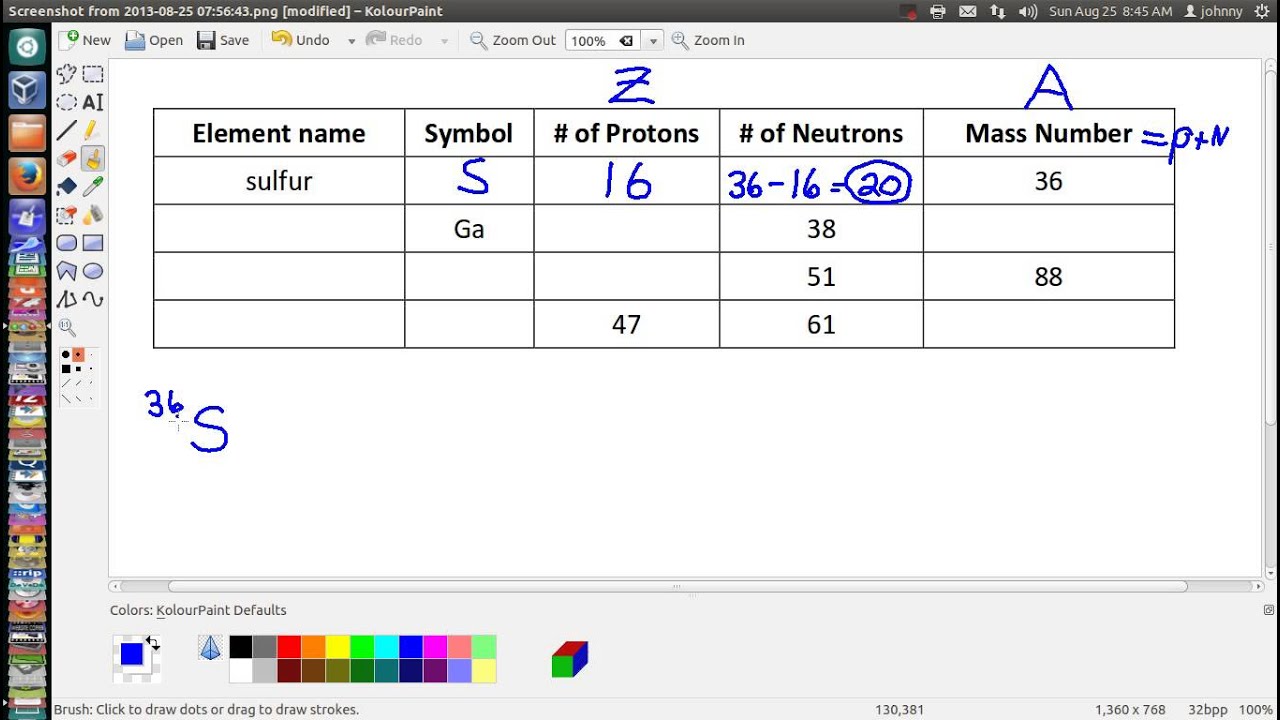
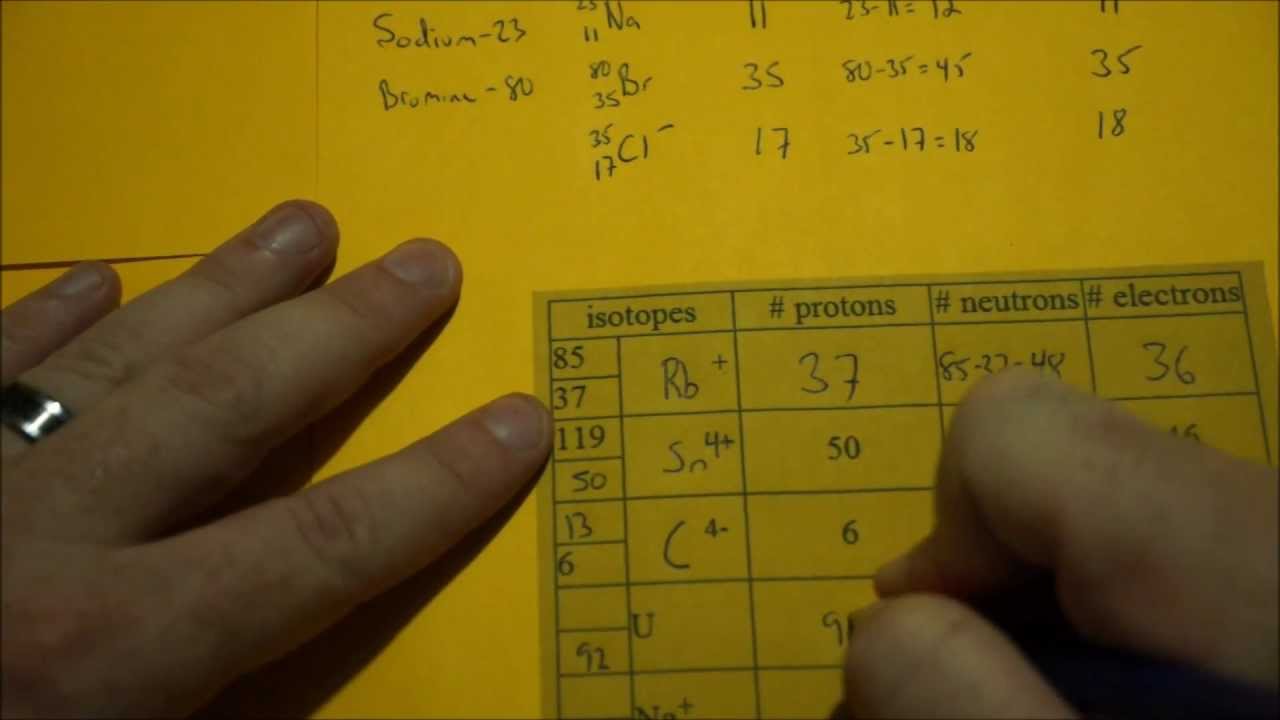
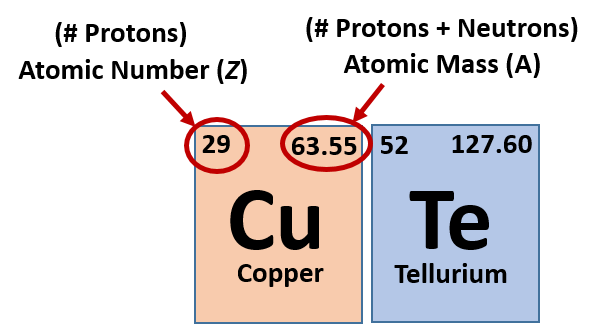
This chemistry video tutorial explains how to calculate the number of protons, neutrons, and electrons in an atom or in an ion. So to get the number of electrons, you must add the size of charge to the atomic or proton number. The number of protons in the nucleus of the atom is equal to the.
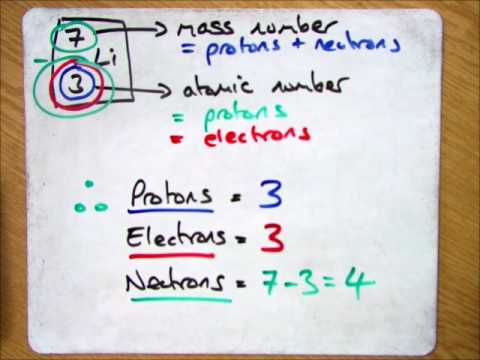
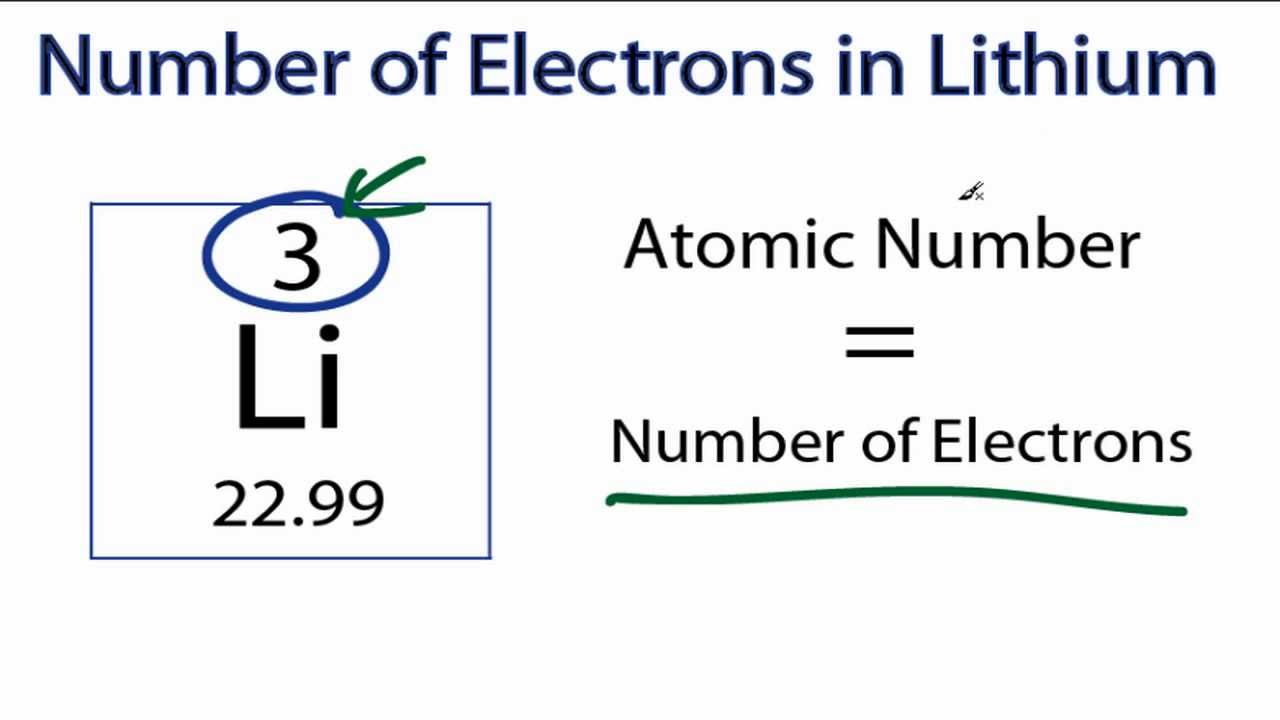
The smallest of the permanent core particles of an atom is the electron. There are as many orbitals in the n shell as the sum of the first mathrm n odd numbers:. The number of electrons in a neutral atom is equal to the number of protons.
Thomson discovered the existence of electrons through cathode ray examination. The initial row of the occasional table has a single. It also explains the differe.
The 1 st subshell (p) can hold 3 orbitals, or 6 electrons.